Ideal Gas Law R Values | The units for r require that the units for pressure must be in atm. A sample of dry gas weighing 3.1134 grams is found to occupy 3.650 l at 22.0°c and 740.0 mmhg. Therefore, the 740.0 mm hg must be converted first. At stp (p = 101 325 pa, t = 273.15 k), the molar volume or volume per mole is 22.414 × 10 −3 m 3 mol −1.therefore, we can calculate the value of r as For example, blood pressure is typically expressed in mm of hg (mercury), as in boyle's experiments.
At stp (p = 101 325 pa, t = 273.15 k), the molar volume or volume per mole is 22.414 × 10 −3 m 3 mol −1.therefore, we can calculate the value of r as A sample of dry gas weighing 3.1134 grams is found to occupy 3.650 l at 22.0°c and 740.0 mmhg. For example, blood pressure is typically expressed in mm of hg (mercury), as in boyle's experiments. May 28, 2019 · value of ideal gas constant in si unit. N refers to the number of moles of gas while r is an ideal gas constant which means that the value is the same for all types of gases.

The units for r require that the units for pressure must be in atm. Often, we write the ideal gas law as: Therefore, the 740.0 mm hg must be converted first. The constant is also a combination of the constants. Pressure is measured in many different units. A sample of dry gas weighing 3.1134 grams is found to occupy 3.650 l at 22.0°c and 740.0 mmhg. N refers to the number of moles of gas while r is an ideal gas constant which means that the value is the same for all types of gases. At stp (p = 101 325 pa, t = 273.15 k), the molar volume or volume per mole is 22.414 × 10 −3 m 3 mol −1.therefore, we can calculate the value of r as The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol r or r. Finally, if the mass and pressure are held constant, the volume is directly proportional to the temperature for an ideal gas. This relationship between pressure and volume is called boyle's law in honor of robert boyle who first observed it in 1660. 740.0 mm hg ÷ 760.0 mm hg/atm = 0.9737 atm. For example, blood pressure is typically expressed in mm of hg (mercury), as in boyle's experiments.
N refers to the number of moles of gas while r is an ideal gas constant which means that the value is the same for all types of gases. 740.0 mm hg ÷ 760.0 mm hg/atm = 0.9737 atm. Nov 20, 2020 · boyle's law, ideal gas, atmospheric pressure, manometer. Often, we write the ideal gas law as: The units for r require that the units for pressure must be in atm.
The units for r require that the units for pressure must be in atm. May 13, 2021 · the product of pressure and volume is exactly a constant for an ideal gas. Pressure is measured in many different units. A sample of dry gas weighing 3.1134 grams is found to occupy 3.650 l at 22.0°c and 740.0 mmhg. 740.0 mm hg ÷ 760.0 mm hg/atm = 0.9737 atm. This relationship between pressure and volume is called boyle's law in honor of robert boyle who first observed it in 1660. How many moles of the gas are present? At stp (p = 101 325 pa, t = 273.15 k), the molar volume or volume per mole is 22.414 × 10 −3 m 3 mol −1.therefore, we can calculate the value of r as The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol r or r.it is the molar equivalent to the boltzmann constant, expressed in units of energy per temperature increment per mole, i.e. Finally, if the mass and pressure are held constant, the volume is directly proportional to the temperature for an ideal gas. For example, blood pressure is typically expressed in mm of hg (mercury), as in boyle's experiments. Nov 20, 2020 · boyle's law, ideal gas, atmospheric pressure, manometer. May 28, 2019 · value of ideal gas constant in si unit.
Nov 20, 2020 · boyle's law, ideal gas, atmospheric pressure, manometer. What assumption is made about the temperature of the gas in this experiment? Pressure is measured in many different units. May 13, 2021 · the product of pressure and volume is exactly a constant for an ideal gas. The constant is also a combination of the constants.
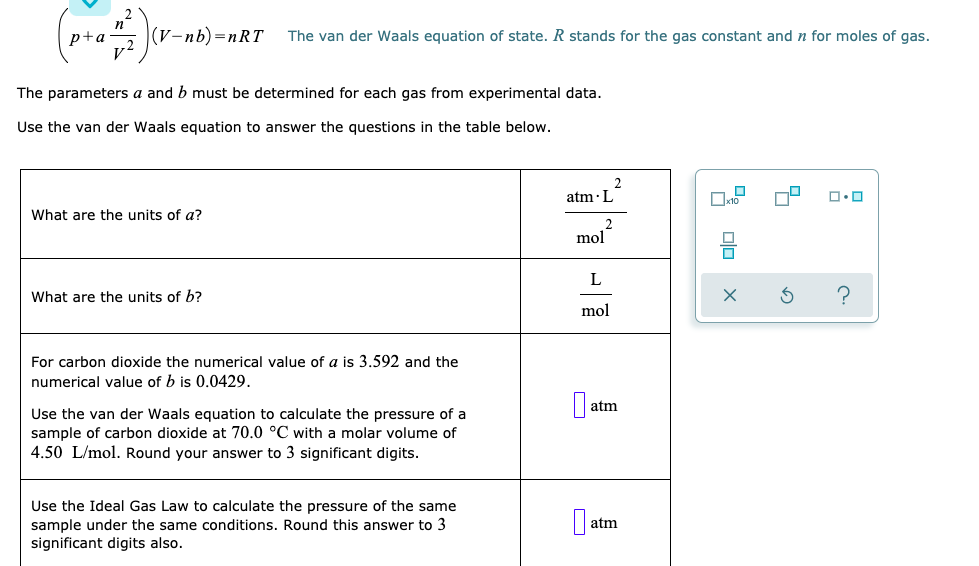
May 28, 2019 · value of ideal gas constant in si unit. How many moles of the gas are present? For example, blood pressure is typically expressed in mm of hg (mercury), as in boyle's experiments. Finally, if the mass and pressure are held constant, the volume is directly proportional to the temperature for an ideal gas. Therefore, the 740.0 mm hg must be converted first. The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol r or r. Pressure is measured in many different units. The constant is also a combination of the constants. The units for r require that the units for pressure must be in atm. Nov 20, 2020 · boyle's law, ideal gas, atmospheric pressure, manometer. The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol r or r.it is the molar equivalent to the boltzmann constant, expressed in units of energy per temperature increment per mole, i.e. May 13, 2021 · the product of pressure and volume is exactly a constant for an ideal gas. It is the molar equivalent to the boltzmann constant, expressed in units of energy per temperature increment per mole, i.e.
Ideal Gas Law R Values: Nov 20, 2020 · boyle's law, ideal gas, atmospheric pressure, manometer.
0 comments:
Post a Comment